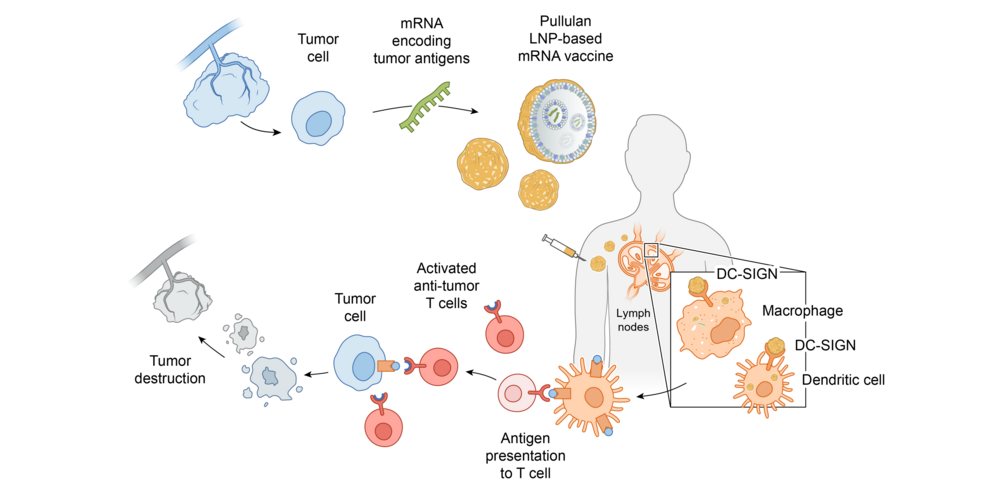
UI-201: Booster vaccine technology for TCR-T cell therapy
We are developing efficient T-cell-inducing vaccines by leveraging an advantage of our Myeloid Targeting Platform™ to target dendritic cells, the key antigen-presenting cells in the lymph node. Recently, we utilized this technology for a novel booster strategy to improve efficacy, safety, and in vivo persistence of anti-tumor TCR-T cells in cancer patients. Gene-engineered T cell therapy such as TCR-T cells or CAR-T cells represent a new therapeutic paradigm for the treatment of cancer. While showing life-saving efficacy of CAR-T cell therapy in hematological cancer, the results in solid tumors have been mixed due to limited in vivo persistence and tumor homing of infused T cells and the hostile microenvironment in cold tumors that causes exhaustion of engineered T cells. The promising strategy to overcome these limitations is to use PNP or P-LNP as a booster for engineered T cells that delivers a cognate tumor antigen to dendritic cells in the lymph node to enhance the proliferation and tumor killing activity of engineered T cells through antigen presentation in a continuous, high-quality manner.
UI-201 consists of a PNP carrying a long peptide antigen derived from a famous shared tumor antigen NY-ESO-1. A First-in-Human clinical study confirmed the promising safety and efficacy of the combination of UI-201 and NY-ESO-1 TCR-T cell therapy for the treatment of refractory solid tumors. Our T-cell booster vaccine technology can be expanded to other tumor antigens including neoantigens.