Myeloid Targeting Platform™
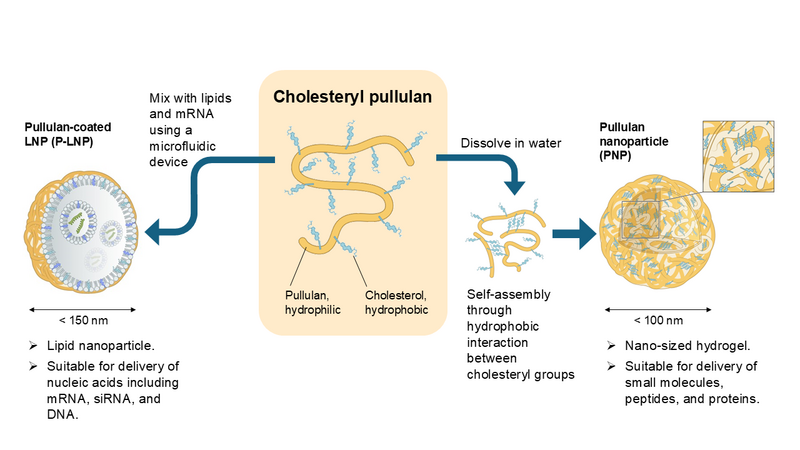
Our Myeloid Targeting Platform™ includes the twins of nanoparticles derived from pullulan polysaccharide: pullulan nanoparticle (PNP) and pullulan-coated, PEG-free lipid nanoparticle (P-LNP). PNP is useful for the delivery of small molecules, peptides, and proteins. P-LNP can encapsulate mRNA, siRNA, and DNA. By using either nanoparticle, almost any therapeutic agents can be delivered to disease- and vaccination-associated, DC-SIGN-expressing myeloid cells selectively.
DC-SIGN-expressing myeloid cells are certain subsets of macrophages and dendritic cells. They are localized in the diseased tissues as well as the normal lymphoid organs. For example, many solid tumors contain DC-SIGN-expressing tumor-associated macrophages (TAMs) abundantly that have strong immune suppressive functions and thereby support immune escape and treatment-resistance of tumor cells. Myeloid Targeting Platform™ can deliver therapeutic agents to these TAMs to manipulate their function, leading to reversal of tumor immune suppression.
Pullulan Nanoparticle (PNP)
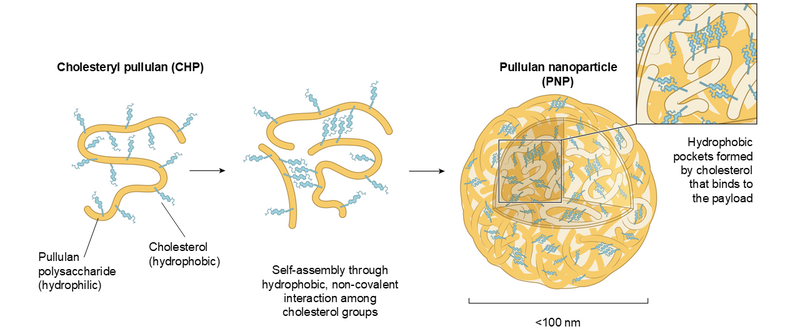
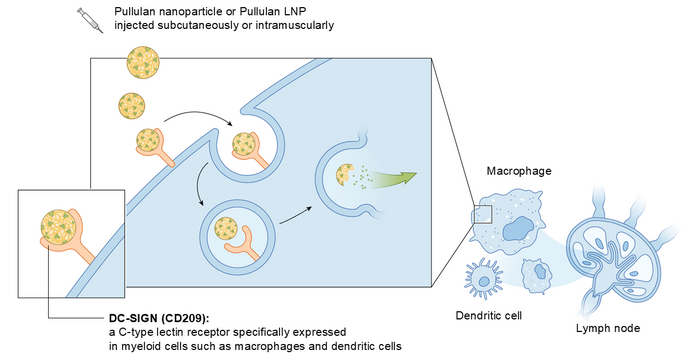
Pullulan Nanoparticle (PNP) is a nanoparticle consisting of cholesterol-modified pullulan polysaccharide. PNP is formed by self-assembly of cholesterol-modified pullulan through hydrophobic interaction between cholesterol groups. Pullulan is a biologically inert polysaccharide isolated from mould and is widely used as an additive for drugs and foods. PNP can encapsulate various payloads including hydrophobic small molecules, peptides and proteins and can be administered by intravenous, subcutaneous, or intramuscular injection. PNP functions as a drug delivery system (DDS) that specifically transport therapeutic payloads to disease- and vaccination-associated, DC-SIGN-expressing macrophages and dendritic cells. This targeting is achieved thought selective binding of pullulan polysaccharide to the sugar-sensing immune receptor DC-SIGN.
Key advantages:
Pullulan Lipid Nanoparticle (P-LNP)
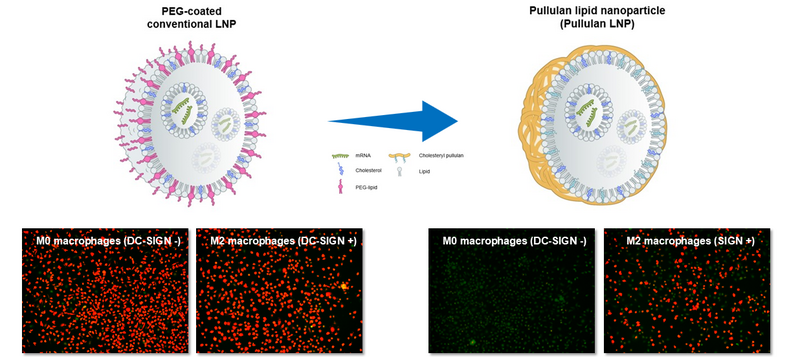
These years, lipid nanoparticle (LNP) is emerging as an important and essential tool in the development of mRNA vaccines against viral infection and cancer as well as mRNA/siRNA drugs. Current LNP technology relies on polyethylene glycol (PEG)-lipid to form LNP and to prevent aggregation. However, PEG-lipid in current LNP can induce anti-PEG antibodies after multiple dosing that can cause some adverse effects such as allergy and anaphylaxis. Anti-PEG antibodies also can cause accelerated blood clearance (ABS) effect that mitigates the efficacy of any PEG-containing vaccines and drugs. In addition, current PEG-based LNP lacks tissue- and/or cell-targeting ability, potentially increasing side effects and limiting efficacy.
We apply our Myeloid Targeting Platform™ to solve these PEG-related issues by inventing next generation LNP so called “pullulan-coated LNP (P-LNP)”. Ideally it contains no PEG-lipid, and pullulan polysaccharide has quite low immunogenicity. By this improvement, we can avoid anti-PEG antibody-associated adverse effects and ABC effects. Simultaneously, pullulan coating of LNP brings selectivity towards vaccination- or disease-associated macrophages and dendritic cells, thereby increases safety and efficacy of LNP products.
Myeloid Specific Cell Receptor DC-SIGN
Targeting of pullulan nanoparticle (PNP) and pullulan-coated lipid nanoparticle (P-LNP) to myeloid cells is achieved by specific binding of pullulan polysaccharide contained in these nanoparticles to a C-type lectin receptor DC-SIGN (also known as CD209) (PAT pending). DC-SIGN is present on the surface of macrophages, dendritic cells, Kupffer cells (liver), Langerhans cells (skin), Hoffbauer cells (placenta), adipose tissue macrophages, and microglia. A physiological role of DC-SIGN is sensing of polysaccharides on pathogens and promotion of their internalization into these cells. DC-SIGN also mediates the contact between antigen-presenting dendritic cells and macrophages to T cells.
In many types of solid tumors, DC-SIGN is predominantly expressed in anti-inflammatory M2-like tumor associated macrophages (TAMs) which promote immune suppression within the tumor microenvironment. Expression of DC-SIGN or frequency of M2-like TAMs are reported to be associated with tumor progression, poor prognosis and treatment resistance to immune checkpoint inhibitors.
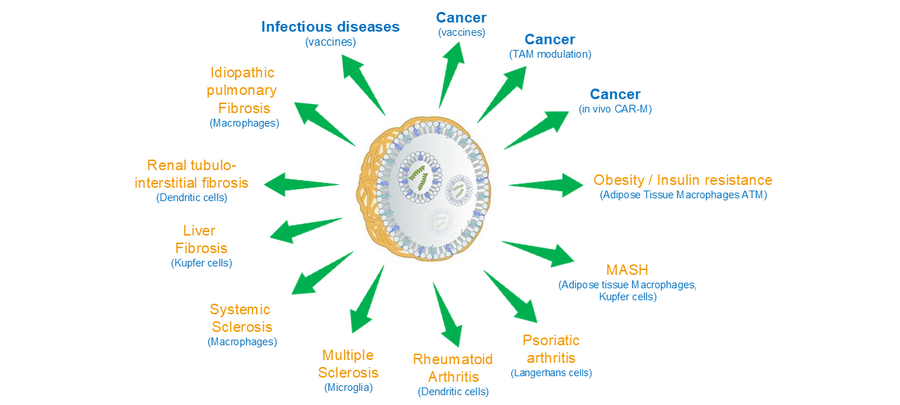
Therapeutic Applications
Recent studies have shown that macrophages and dendritic cells are directly involved in many major diseases including cancer, fibrosis, viral infection, inflammation, neurodegeneration, and autoimmune diseases. In human solid tumors, DC-SIGN expression is observed in many tumor types such as liver cancer, bladder cancer, renal cell cancer, gastric cancer, head and neck cancer, and lung cancer. These cancers are potential indications for novel immunotherapies utilizing our Myeloid Targeting Platform™. In addition to solid tumors, DC-SIGN expression in macrophages has also been reported in idiopathic pulmonary fibrosis (IPF), renal tubulointerstitial fibrosis, systemic sclerosis, rheumatoid arthritis, psoriatic arthritis, viral infection, obesity, nonalcoholic fatty liver disease (NAFLD), insulin resistance, and others. Although further preclinical and clinical investigation is required, DC-SIGN-expressing myeloid cells also have significant roles in these non-cancer diseases. Selective targeting of PNP and P-LNP to DC-SIGN-expressing macrophages and dendritic cells in these pathogenic conditions offers the potential of delivering therapeutic payloads addressing these diseases.
In the healthy condition, human DC-SIGN is expressed in dendritic cells localizing in the important lymphoid organs such as the lymph node, skin, and spleen. Dendritic cells in these organs plays a central role in antigen presentation and efficient induction of anti-viral or anti-cancer immune response after vaccination. Myeloid Targeting Platform™ is therefore also useful for the efficient delivery of vaccine antigen to dendritic cells in these lymphoid organs. Indeed, PNP has been evaluated as a vaccine delivery system in a series of cancer peptide/protein vaccines in the clinical.