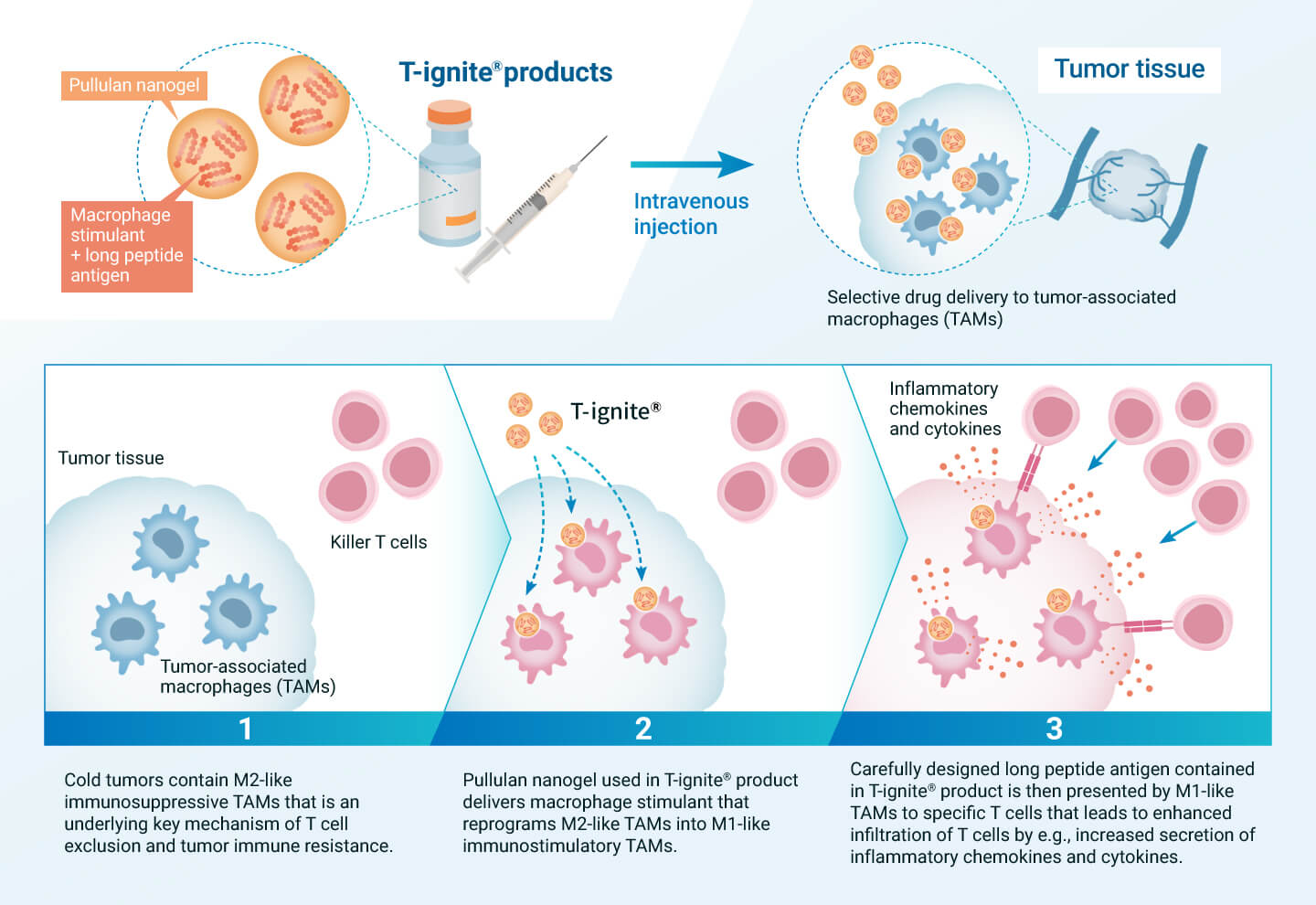
Activating Immunity by Acting on Macrophages “Inside” Cancer
If the functions of tumor-associated macrophages, which are the cause of cold tumors, can be successfully regulated, it is expected that treatment-resistant cold tumors can be converted into treatment-sensitive hot tumors, leading to therapeutic effects on intractable cancers. To achieve this, we are developing pullulan nanogel-based T-ignite® for intravenous administration, which is loaded with various therapeutic components that act on tumor-associated macrophages.
T-ignite® administered intravenously is selectively taken up by M2-like immunosuppressive macrophages in the tumor tissue through the interaction of pullulan nanogel with DC-SIGN. The drug contained in T-ignite® then modulates the function of macrophages, reprogramming them from an immunosuppressive M2 type that interferes with T cells to an immunostimulatory M1 type that supports T cells. This is believed to activate immunity from within the cancer tissue and convert the cancer to treatment sensitivity. T-ignite® for intravenous administration would be compatible with other immunotherapies that assist T-cell function, especially immune checkpoint inhibitors. We will develop a series of diverse T-ignite® products depending on the type of cancer and the drug to be loaded.
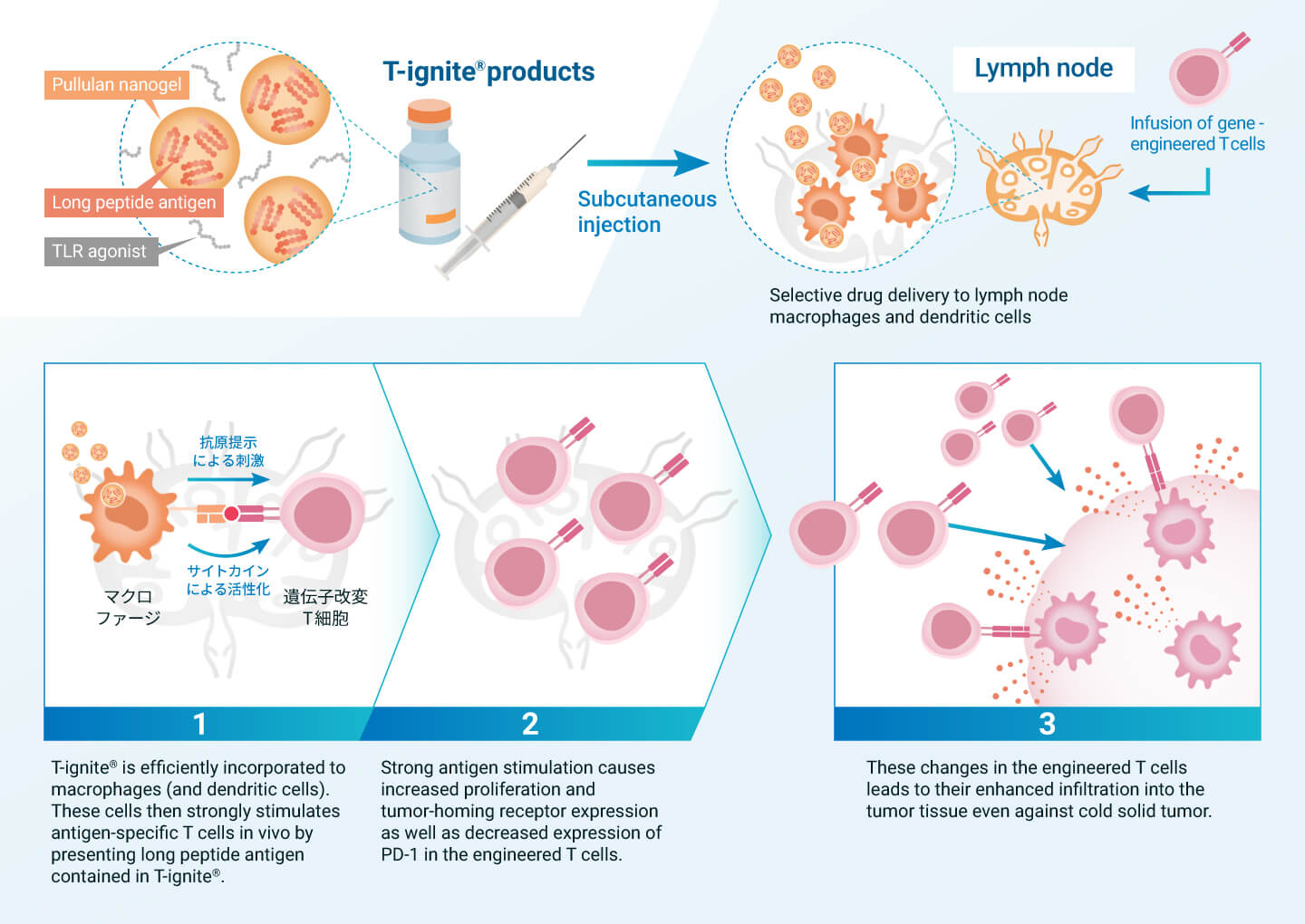
Also Activating Macrophages “Outside” Cancer to Boost Anti-Tumor Immunity
Immune resistant cancer is characterized by the lack of sufficient number of tumor-specific T cells in the patient’s body. To overcome this situation, chimeric antigen receptor (CAR) gene-modified (CAR-T) and T-cell receptor (TCR) gene-modified (TCR-T) T-cell therapies have been developed. These therapies involve the administration of large numbers of genetically modified and amplified T cells outside the patient’s body as a therapeutic agent. Although they are powerful immunotherapies, they are not effective against solid tumors, especially cold tumors that do not attract T cells.
T-ignite® products have the potential to enhance the performance of these genetically engineered T-cell therapies and evolve them into effective treatments for cold tumors as well. T-ignite® can provide high-quality antigen stimulation to genetically modified T cells through lymph node macrophages and dendritic cells. As a result, the genetically modified T cells continue to amplify in the body and acquire the ability to invade cancer tissue. In collaboration with universities, we conducted a Phase 1 clinical trial of the combination therapy of T-ignite® and TCR-T therapy in intractable solid tumor patients and confirmed a promising safety and efficacy profile. We also plan to study combination therapy with CAR-T in the future.